
NH3 Molecular Geometry Science Education and Tutorials
Steps of drawing NH3 lewis structure Step 1: Find the total valence electrons in NH3 molecule. In order to find the total valence electrons in NH3 molecule, first of all you should know the valence electrons present in nitrogen atom as well as hydrogen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
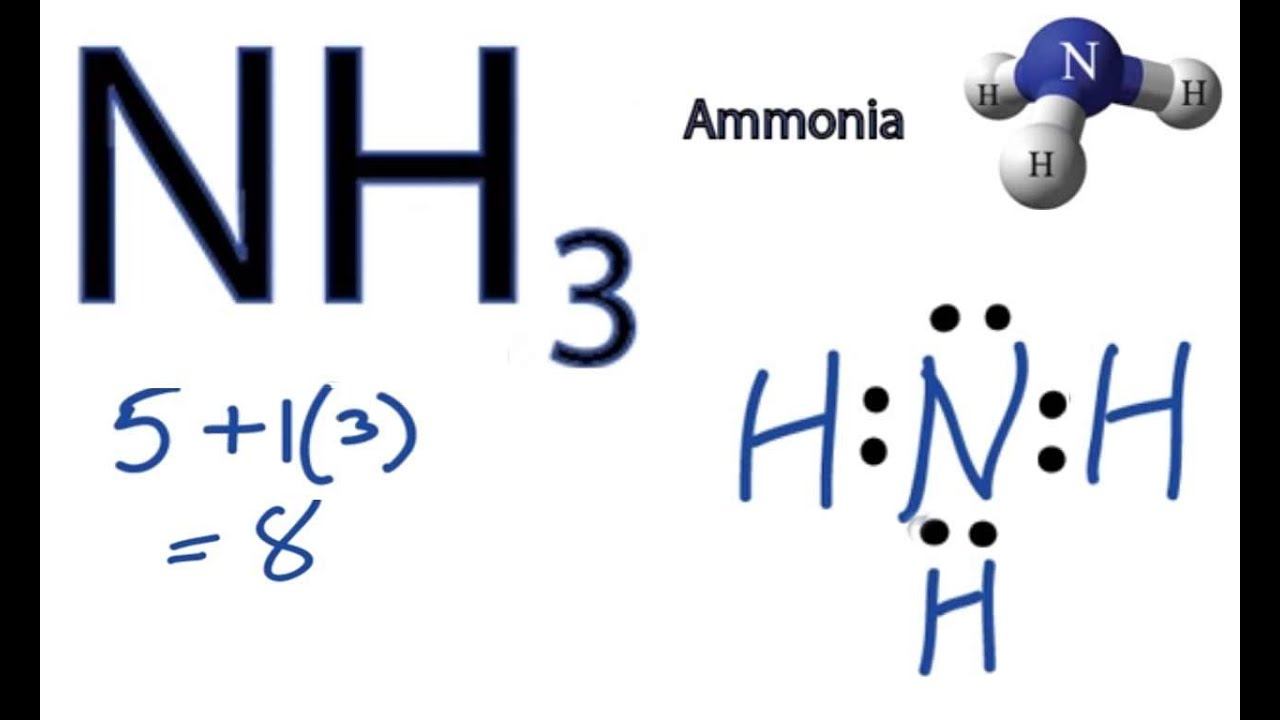
Lewis Dot Diagram For Nh3 exatin.info
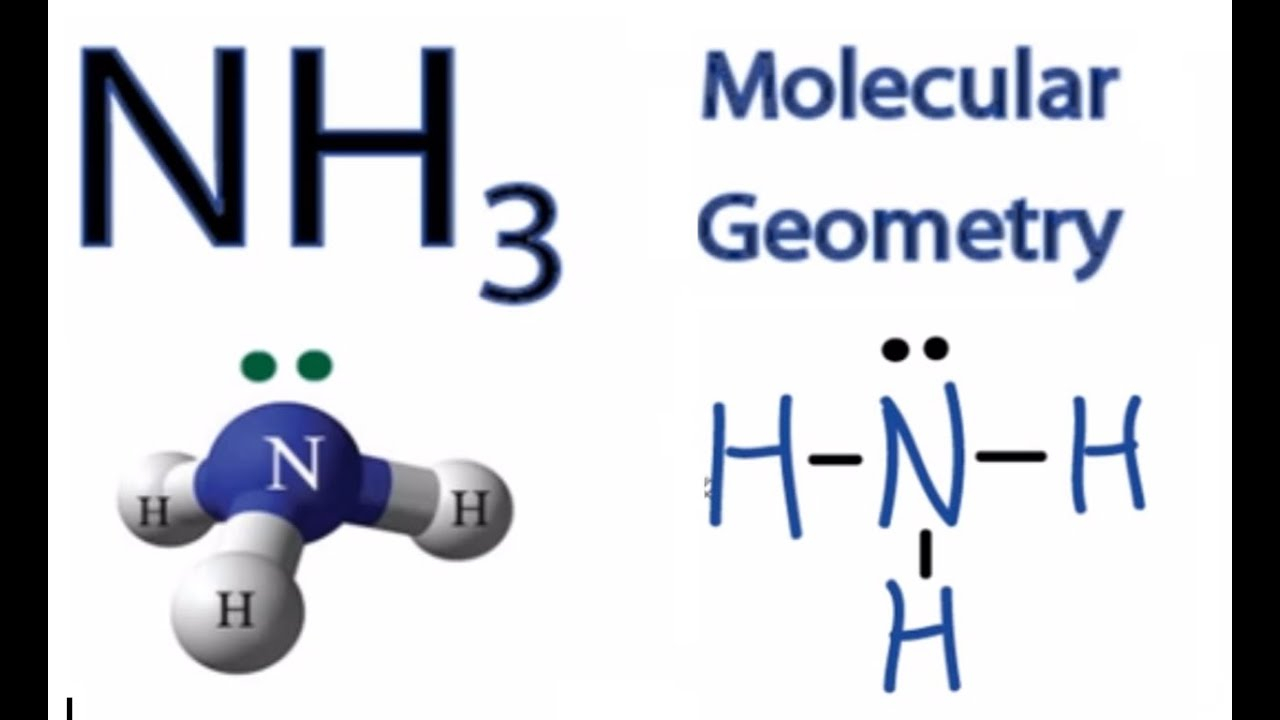
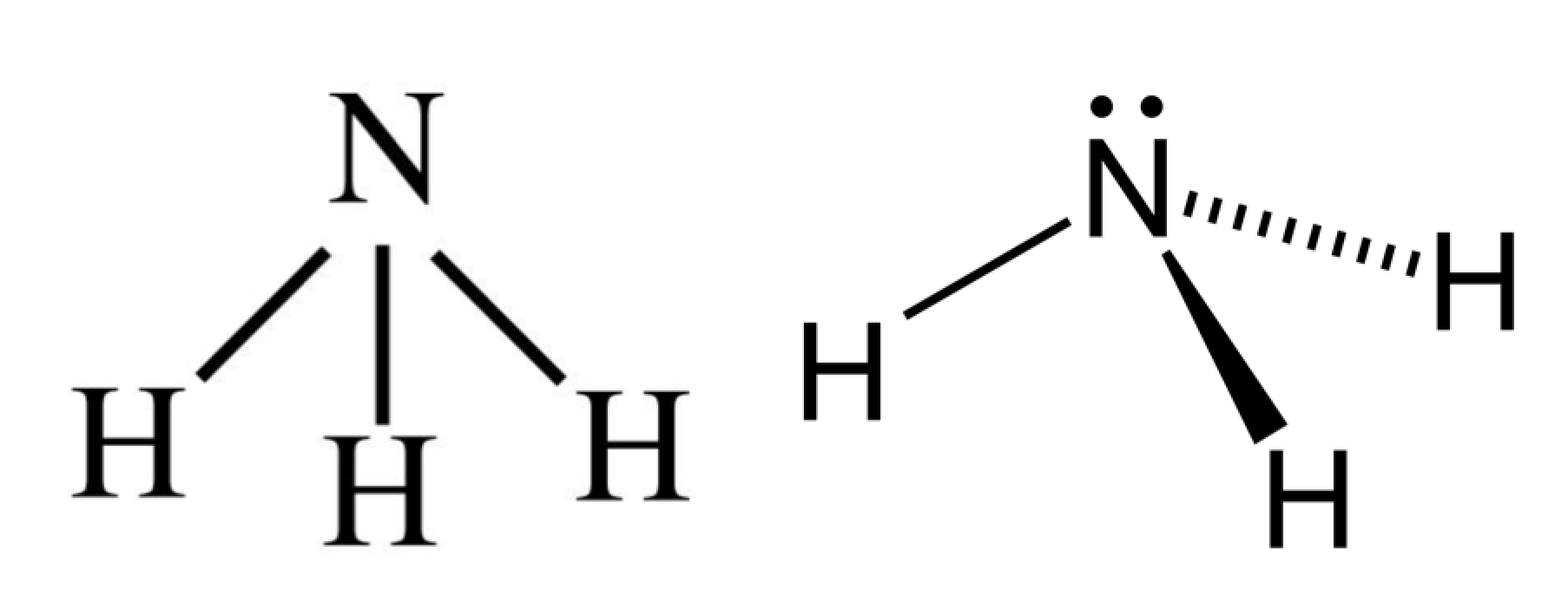
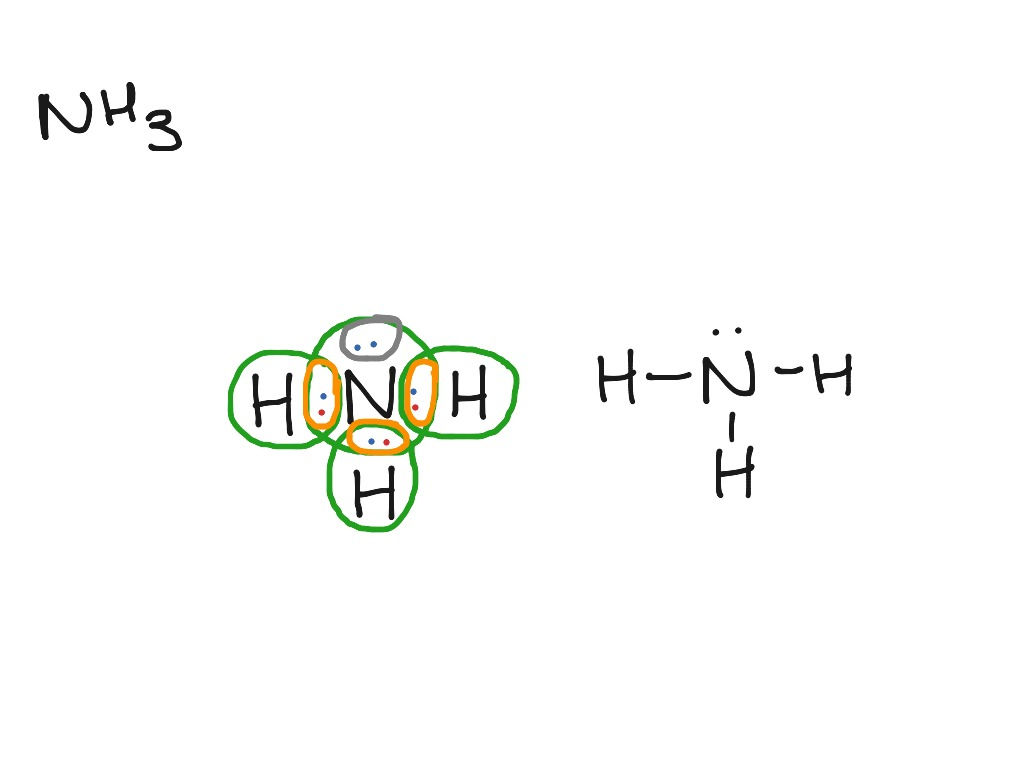
Lewis structure of NH3 (ammonia) contains three single bonds between the Nitrogen (N) atom and each Hydrogen (H) atom. The Nitrogen atom (N) is at the center and it is surrounded by 3 Hydrogen atoms (H). The Nitrogen atom has one lone pair. Let's draw and understand this lewis dot structure step by step.

NH3 (ammonia) Lewis dot structure YouTube
To begin drawing the NH3 Lewis structure, start by counting the total number of valence electrons. Valence electrons are the outermost electrons of an atom and are involved in bonding. For NH3, nitrogen (N) is in Group 5A (Group 15), so it has five valence electrons.

electron dot structure of NH3 Science Carbon and its Compounds
NH3 Lewis Structure: Drawings, Hybridization, Shape, Charges, Pair and Detailed Facts By Lambda Geeks NH3 Lewis structure needs to be described for illustrating the knowledge about the compound. This article would be developed with the facts delivered by the Lewis structure of NH3. The facts that would be emerged in this article are:
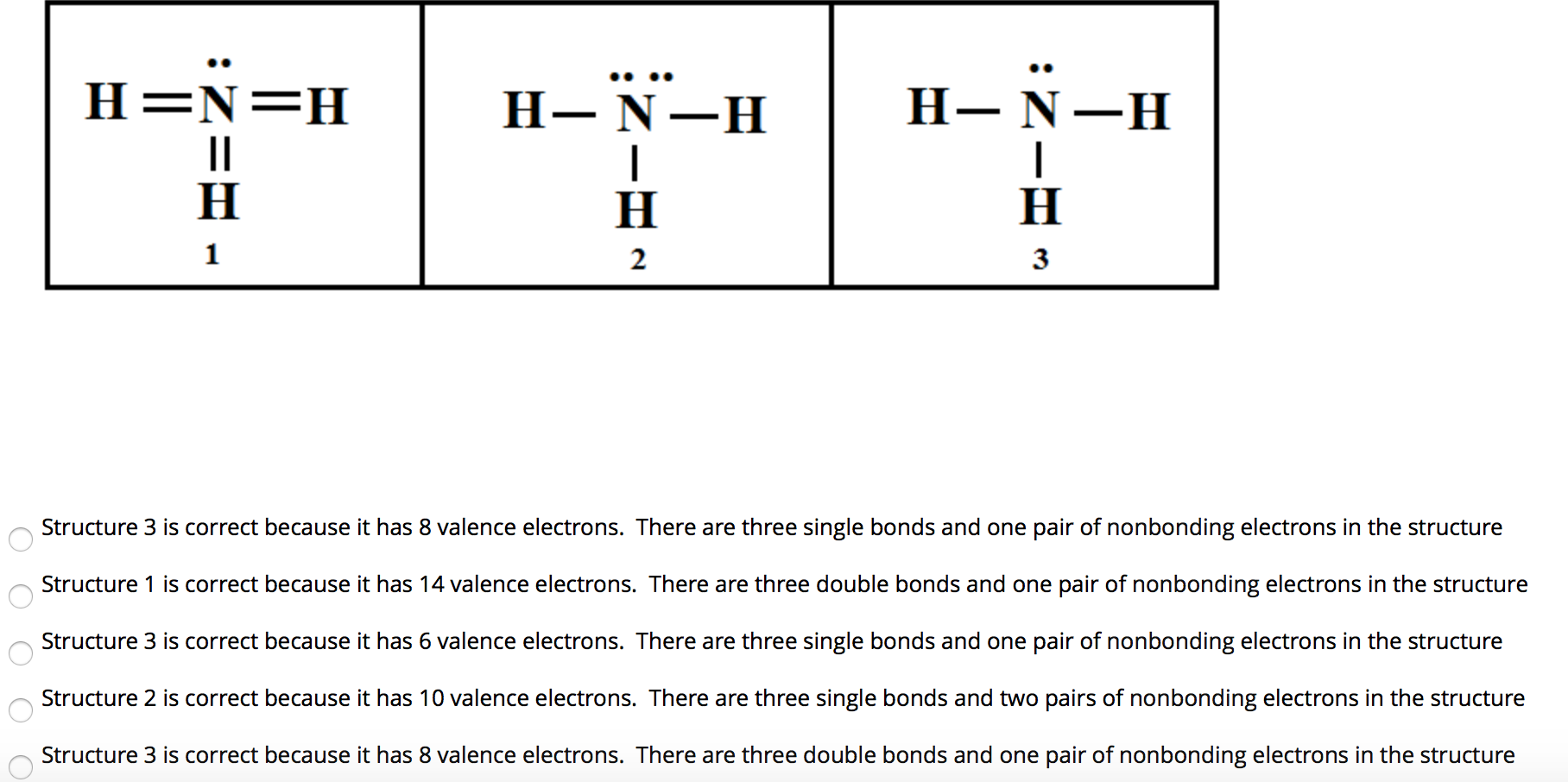
Solved H = N = H HNH H N H Structure 3 is correct
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
MakeTheBrainHappy Is NH3 Polar or Nonpolar?
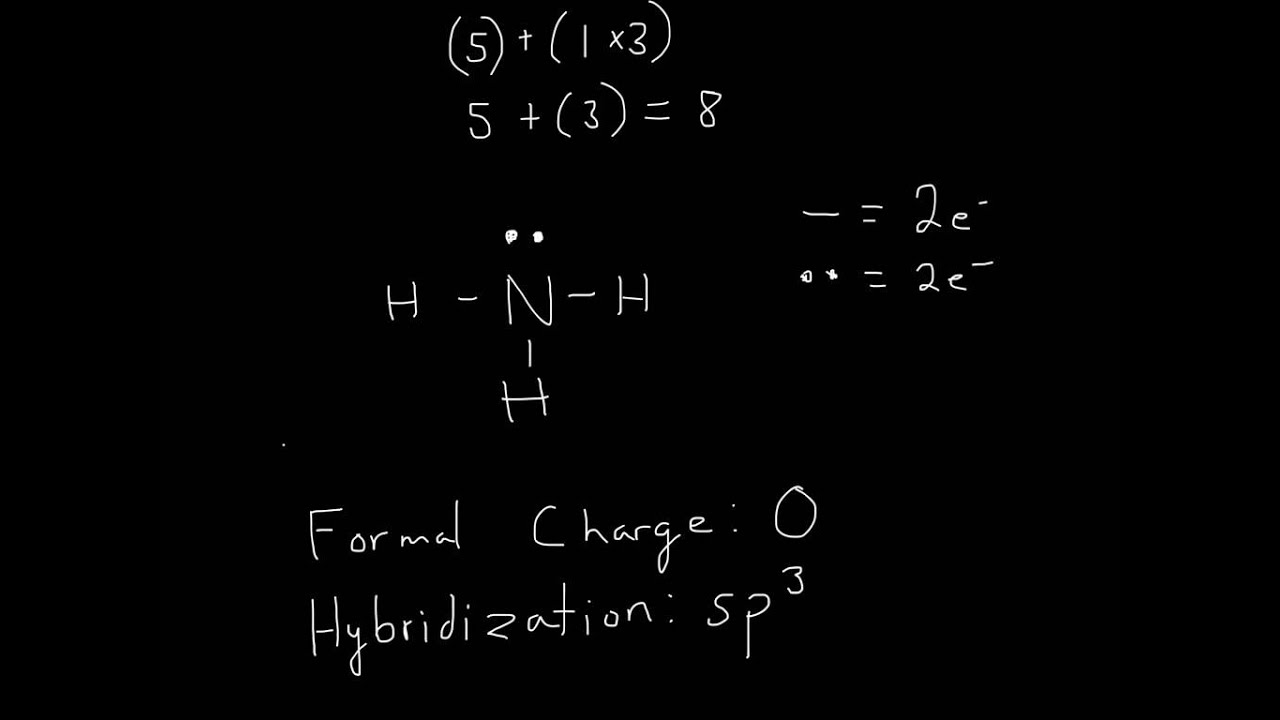
The Lewis structure of nitrogen and hydrogen atom shows a total of eight valence electrons participating in a bond formation, to produce a single tetra-atomic NH3 molecule. Here, we need to study how the Lewis structure of the NH3 molecule is drawn: Search the total number of valence electrons: It is eight to form a single NH3 molecule.
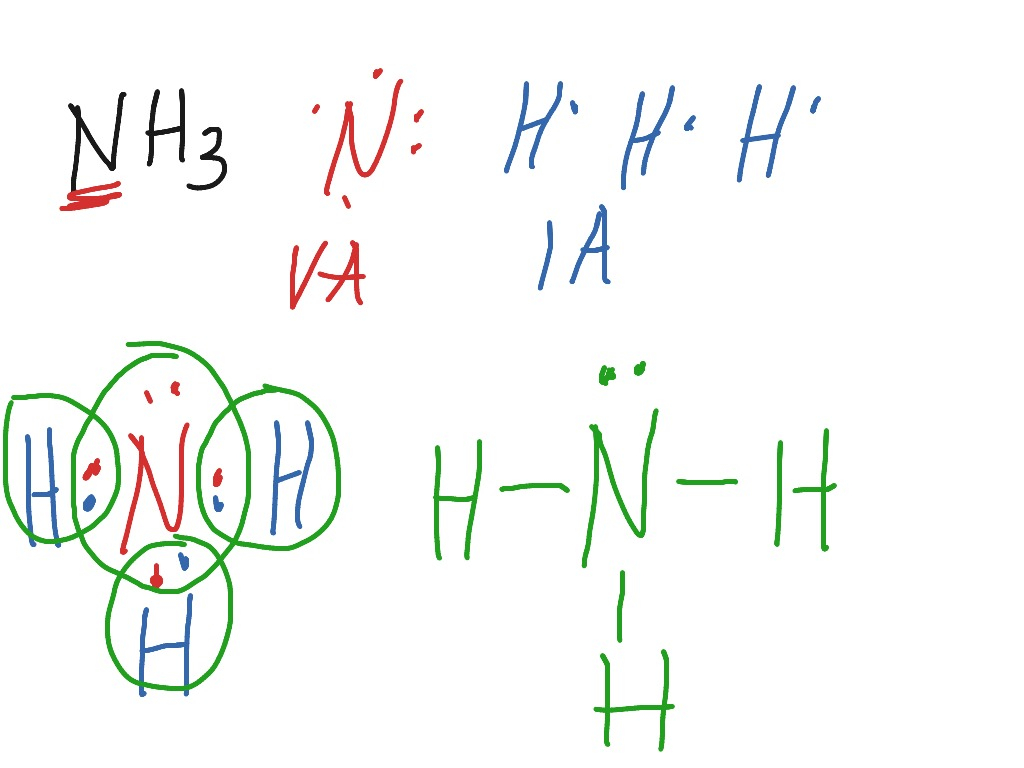
Lewis Dot Diagram For Nh3 exatin.info
In the NH3 lewis dot structure, there are Four atoms present, one N and three H atoms. The valence electrons for N are 5 and for three H atoms are 3. So, the total valence electrons are 5+3 = 8. Step 2 - According to the octet rule, the electrons that will be needed for the NH3 lewis dot structure will be 8 + (3*2) = 14.

Estructura De Lewis Del Amoníaco Nh3 Explicacion Facil Mobile Legends
A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale.
Lewis Dot Diagram For Nh3 exatin.info
This chemistry video tutorial explains how to draw the lewis structure of NH3 also known as Ammonia.Chemistry - Basic Introduction: https:.
Lewis Dot Diagram For Nh3 exatin.info
In the lewis structure of ammonia (NH 3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH 3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Each step of drawing the lewis structure of NH 3 is explained in detail in this tutorial.
Lewis Structure of NH3 YouTube
1: Structure and Bonding 1.3: Lewis Structures Expand/collapse global location 1.3: Lewis Structures Page ID Using Lewis Dot Symbols to Describe Covalent Bonding

Is NH3 Polar or Nonpolar?Is Ammonia a Polar or Nonpolar Molecule?
Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3. Video: Drawing the Lewis Structure for NH3 It is helpful if you:
3d nh3
There is really only one way to draw the Lewis structure for Ammonia (NH3). For resonance structures there must be a double or triple bond present, which is.
Lewis Dot Diagram For Nh3 exatin.info
In the NH 3 Lewis structure, there are three single bonds around the nitrogen atom, with three hydrogen atoms attached to it, and on the nitrogen atom, there is one lone pair. NH3 Lewis Structure - How to Draw the Dot Structure for NH3 (Ammonia) Watch on Contents Steps #1 Draw a rough sketch of the structure

Lewis Dot Diagram Of Nh3
The NH3 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the NH3 molecule. The geometry of the NH3 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory), which states that molecules will choose the NH3 geometrical shape in which the electrons have from one another.
Lewis Dot Diagram For Nh3 exatin.info
The Lewis structure of ammonia, NH_3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.